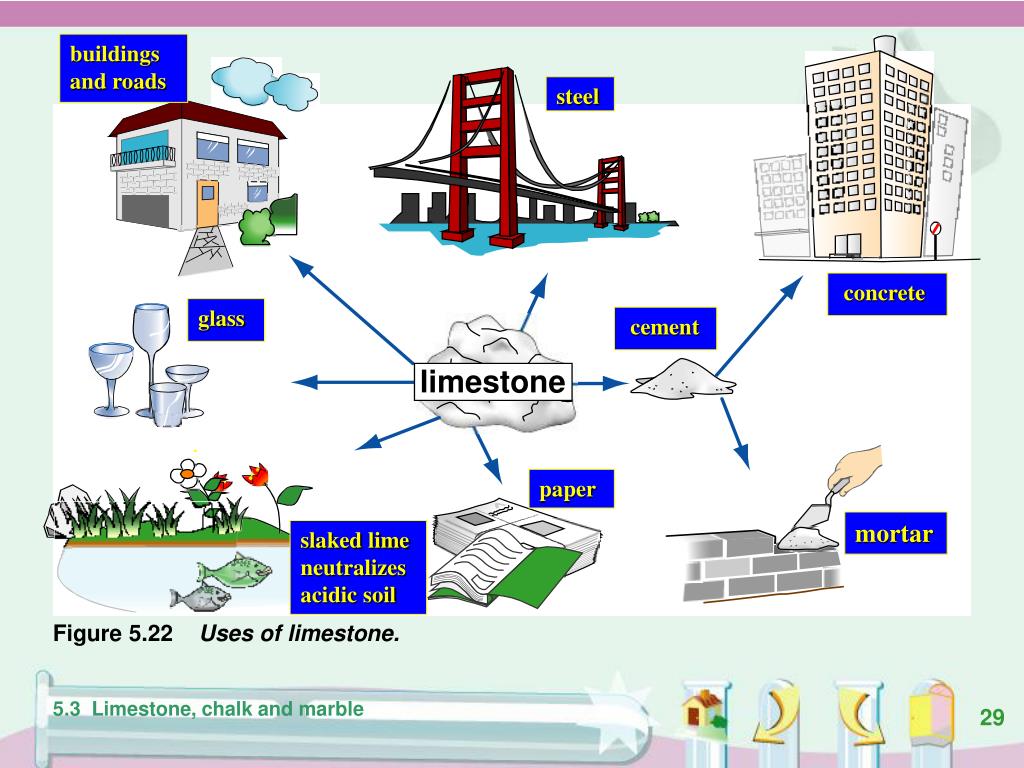
In the lime industry limestone is a general term for rocks that contain 80 or more of calcium or magnesium carbonate including marble chalk oolite and marl further classification is done by composition as high calcium argillaceous clayey silicious conglomerate magnesian dolomite and other limestones.
Marble chalk limestone slaked lime.
By slaking lime with water one obtains naturally slaked lime.
So making slaked lime is definitely something modern man can accomplish.
Chalk is a form of limestone.
Quicklime or burnt lime is heated to about 1000 degrees celsius to form a simplified chemical structure doing away with unwanted carbon dioxide caught up in limestone s molecules.
First off understand that there are two types of lime quicklime and hydrated lime.
Simply put limestone is sometimes processed heated and hydrated and is then termed quick and slaked lime respectively.
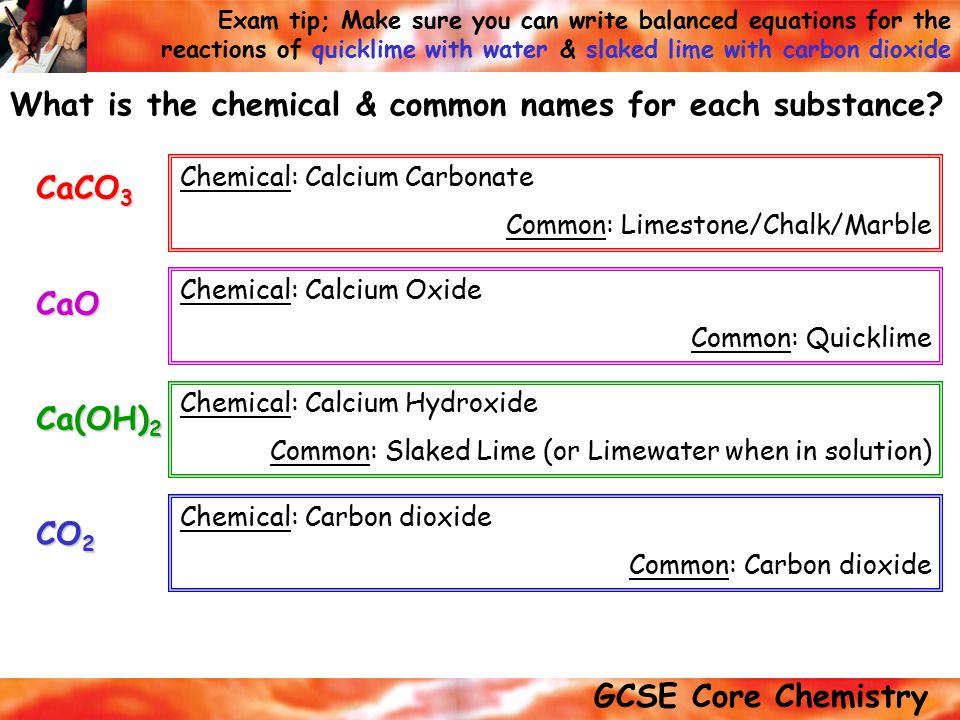
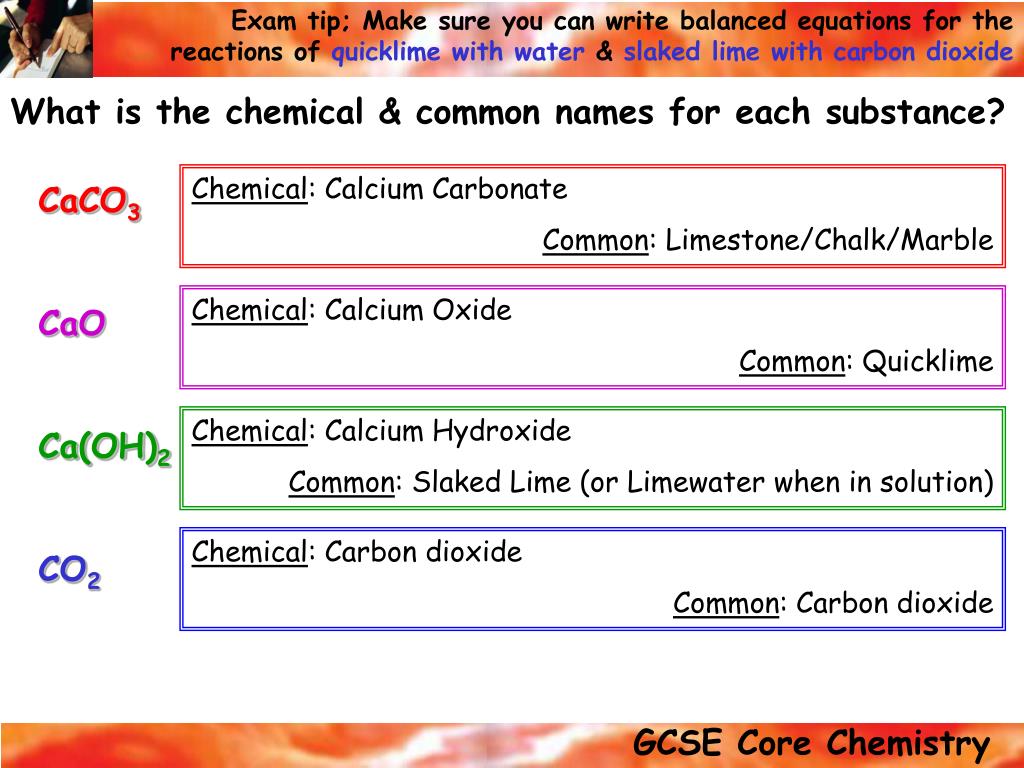
Slaked lime has the chemical formula ca oh 2.
Whitewash or calcimine kalsomine calsomine or lime paint is a type of paint made from slaked lime calcium hydroxide ca oh 2 or chalk calcium carbonate caco 3 sometimes known as whiting various other additives are sometimes used.
The slaking of lime is written in shorthand cao h 2 o ca oh 2 δ.
Quicklime is made by heating calcium carbonate limestone marble chalk shells etc to a temperature of around 1000 c for several to burn or calcimine it.
The triangle or delta symbol indicates heat.
The key difference between limestone and chalk is that the limestone contains both minerals calcite and aragonite whereas chalk is a form of limestone which contains calcite.
Uncommon sources of lime include coral sea shells calcite and.
Therefore this mineral is highly alkaline.