Marble chips calcium carbonate caco 3 react with hydrochloric acid hcl to produce carbon dioxide gas.
Marble chips and hydrochloric acid experiment conclusion.
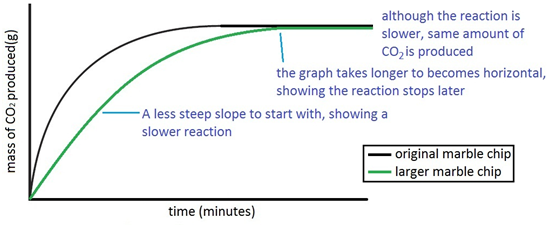
Using the apparatus shown the change in mass of carbon dioxide can be measure with time.
Calcium chloride solution is also formed.
Marble chips react with dilute hydrochloric acid to produce carbon dioxide gas.
Investigating the rate of reaction between marble chips calcium carbonate and hydrochloric acid aim.
Hydrochloric acid marble chips the experiment the aim of this experiment is to find out how different variables affect the rate at which the reaction between marble chips caco and hydrochloric acid hcl takes place.
Hydrochloric acid to react with the marble chips independent variable marble chips to react with the acid dependent variable stopwatch to accurately time the experiment spatula to handle the marble chips measuring cylinder to precisely measure out different concentrations of hydryochloric acid electric balance to measure the mass g of the marble chips bung.
We are going to do an experiment to see how surface area effects the rate of reaction when added to hydrochloric acid.
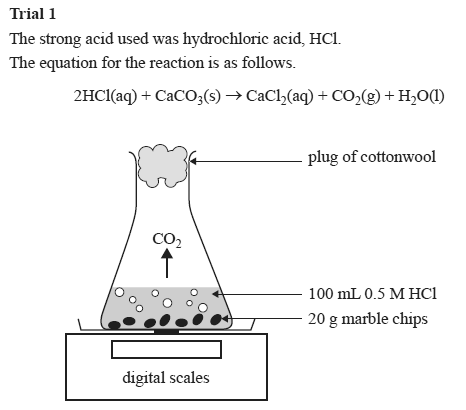
A conical flask contains the marble chips hydrochloric acid and the water that will make the reaction.
When calcium carbonate is added to hydrochloric acid a reaction takes place.
I will add calcium carbonate marble chips to hydrochloric acid.
A tube to connect the conical flask to the measuring cylinder.
A stand to hold up the measuring cylinder.
An investigation of the reaction between marble chips and hydrochloric acid.
There are many variables that affect.
5g of medium sized marble chips with 10cm of 0 25m hydrochloric acid time seconds volume of gas released cm 10 20 30 40 50 60 70 80 90 100 110 120 130 140 150 160 170 180 this table shows that 10cm of hydrochloric acid is an.
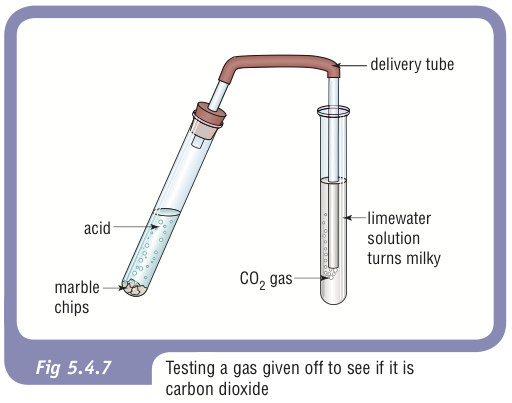
The solution fizzes and gives off the gas carbon dioxide.
The variables that i shall be changing will be the concentration of hydrochloric acid and water.