To investigate the effect of concentration on the reaction between marble chips and hydrochloric acid materials.
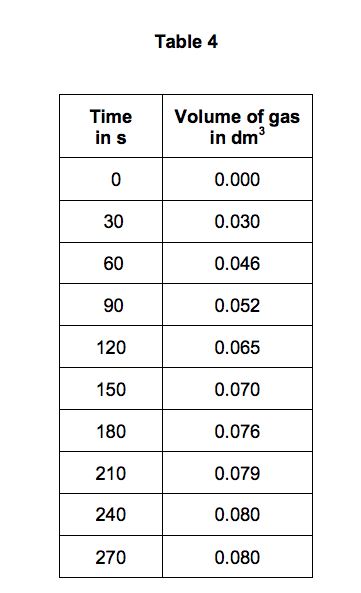
Marble chips and hydrochloric acid experiment graph.
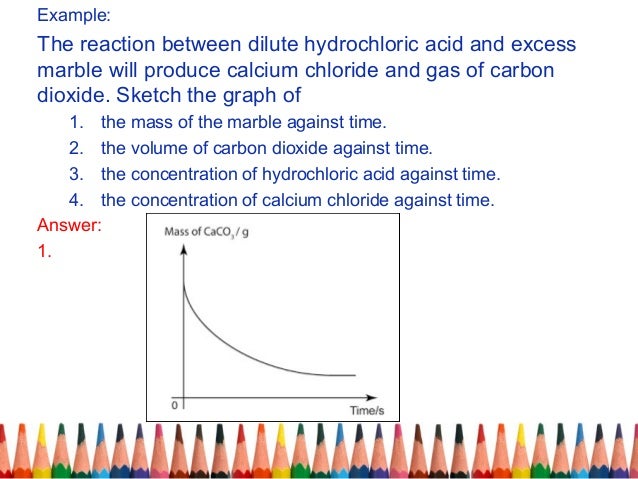
This will not make any difference to the graph as it is the marble chips that are used up not the acid so the graph will be the same.
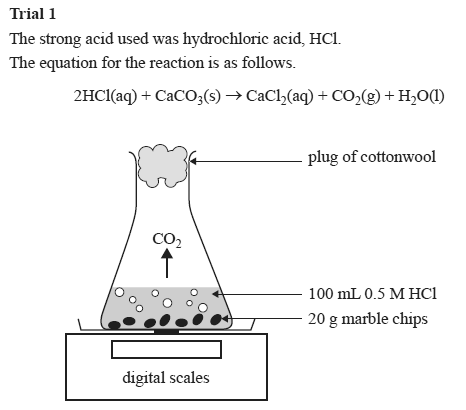
Using the apparatus shown the change in mass of carbon dioxide can be measure with time.
In the reaction between calcium carbonate marble chips and hydrochloric acid we can use the apparatus below to find the rate of reaction.
An investigation of the reaction between marble chips and hydrochloric acid.
Marble chips calcium carbonate caco 3 react with hydrochloric acid hcl to produce carbon dioxide gas.
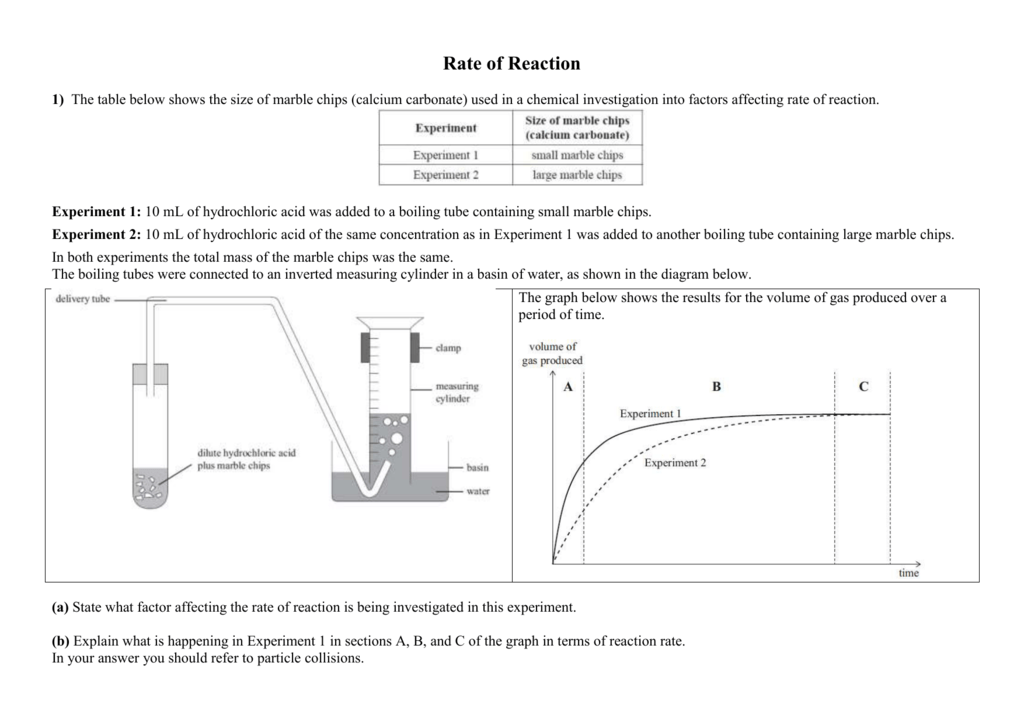
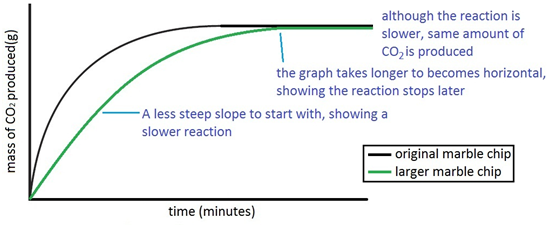
In the investigation i am going to find out how the surface area affects the rate of reaction by measuring the amount of gas produced and weight loss in a reaction between small large pieces of marble chips calcium carbonate and hydrochloric acid per minute.
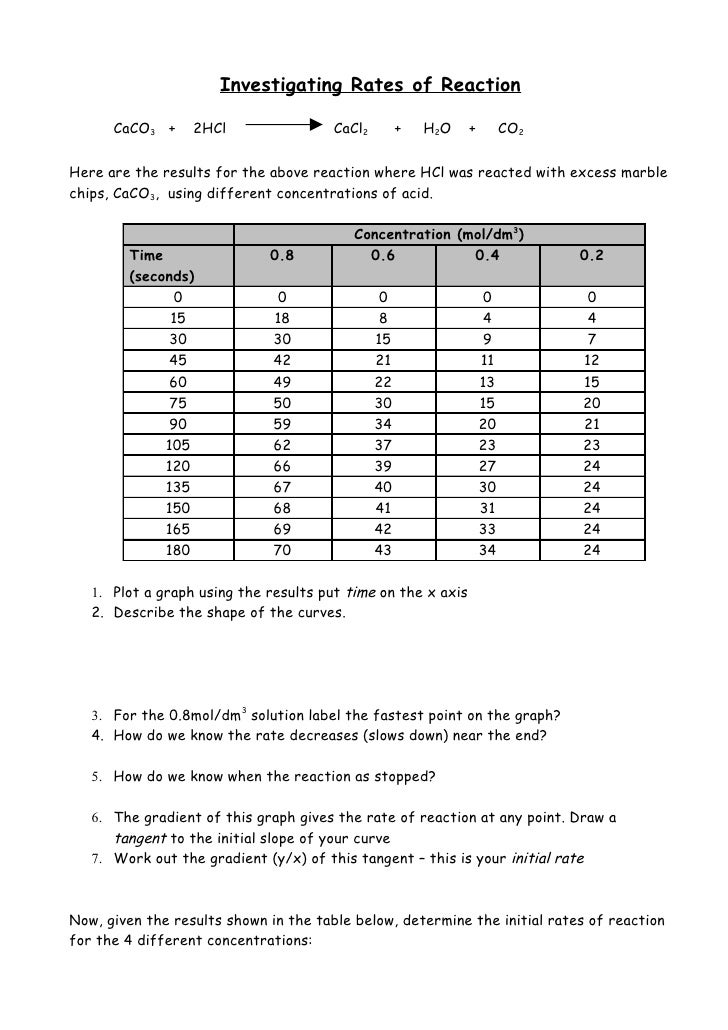
Time s on the horizontal axis.
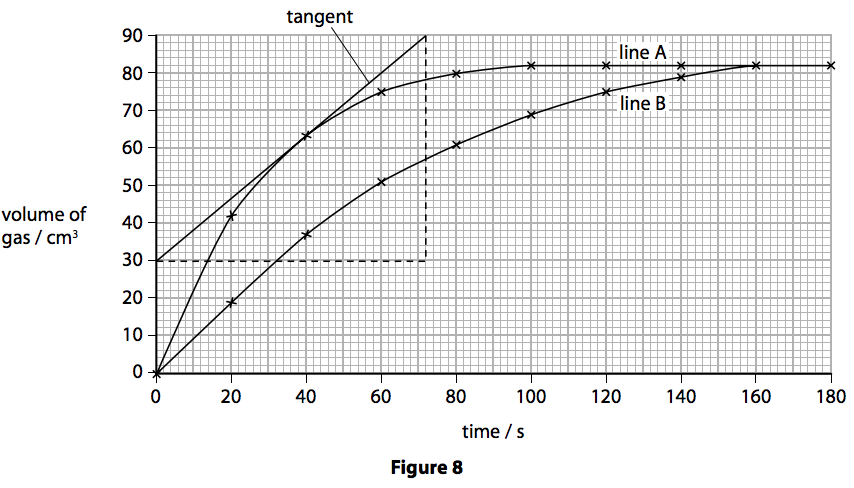
The graph which is most likely to be produced from experiment 3 is graph a the only difference in the conditions compared with experiment 1 is that there is twice as much acid.
Hydrochloric acid marble chips the experiment the aim of this experiment is to find out how different variables affect the rate at which the reaction between marble chips caco and hydrochloric acid hcl takes place.
The rate of this reaction can be changed by changing the size of the marble chips.
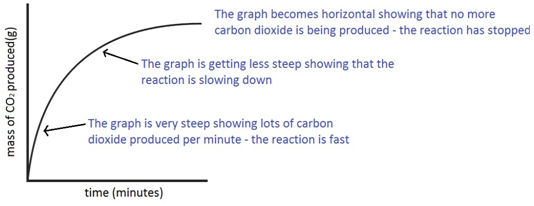
This is because as time goes on the volume of the gas evolved does not change.
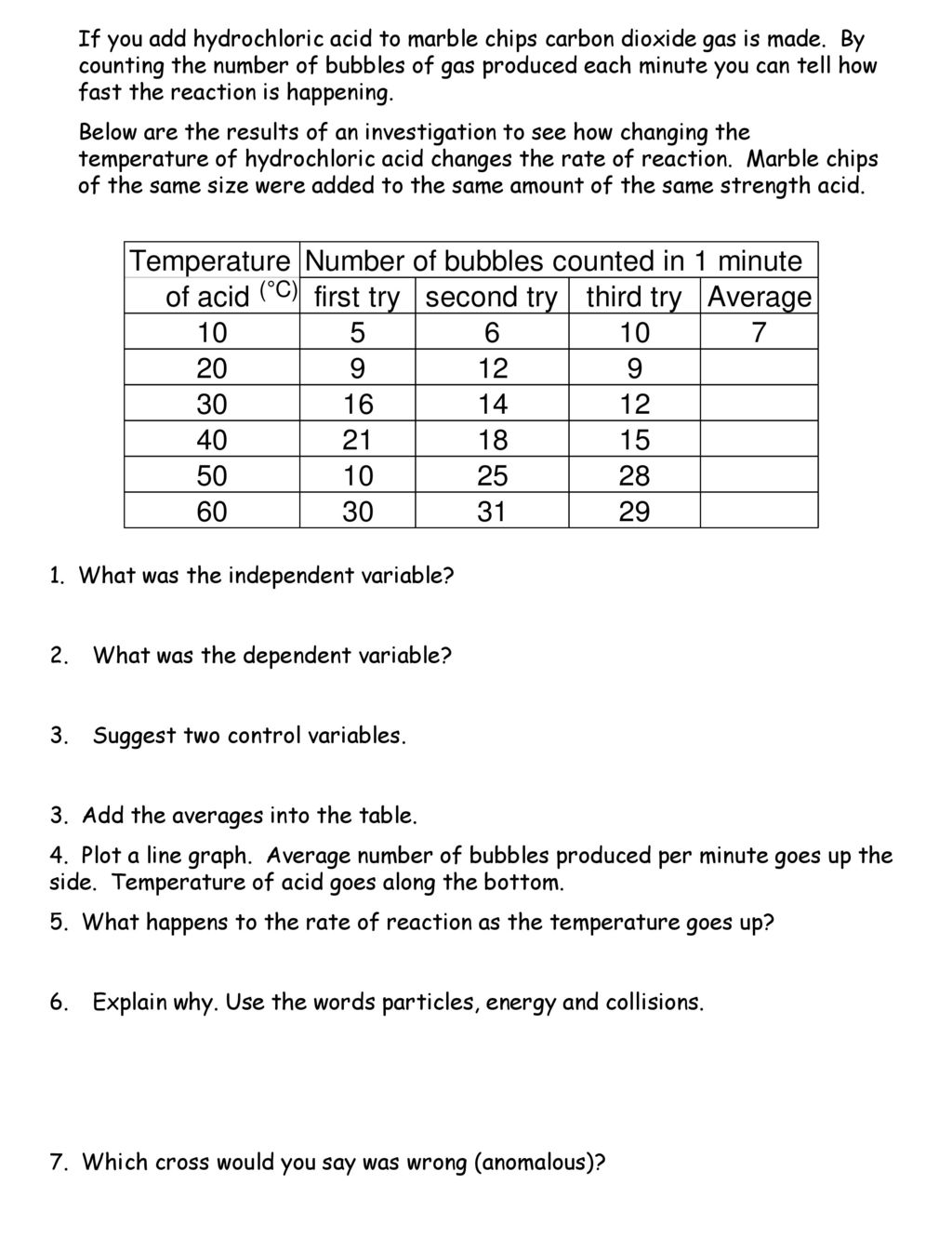
Dgfhhdhdinvestigating rates of reaction aim the aim of my investigation is to find out how rates of reaction are affected by temperature specifically how the rate of the reaction between hydrochloric acid and marble chips is affected by the temperature of the hydrochloric acid.
There are many variables that affect.
For each concentration of hydrochloric acid plot a graph to show.
Draw a curve of best fit.
The essay on hydrochloric acid and marble reactions.
The curve on the graph goes flat when the reaction is complete.
Investigating the rate of reaction between marble chips calcium carbonate and hydrochloric acid aim.
Calcium chloride solution is also formed.
The graph which is most likely to.
Conical flask glass jam jars measuring cylinder stop clock watch with seconds stop watch app direct reading balance dilute hydrochloric acid cotton wool marble chips method.
Hydrochloric acid to react with the marble chips independent variable marble chips to react with the acid dependent variable stopwatch to accurately time the experiment spatula to handle the marble chips measuring cylinder to precisely measure out different concentrations of hydryochloric acid electric balance to measure the mass g of the marble chips bung.
Measuring the rate of loss of a gaseous product.